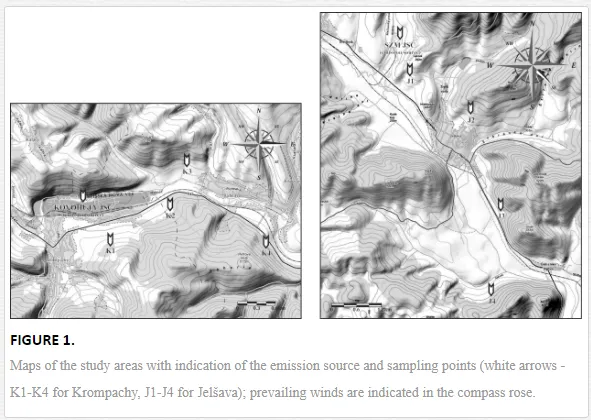
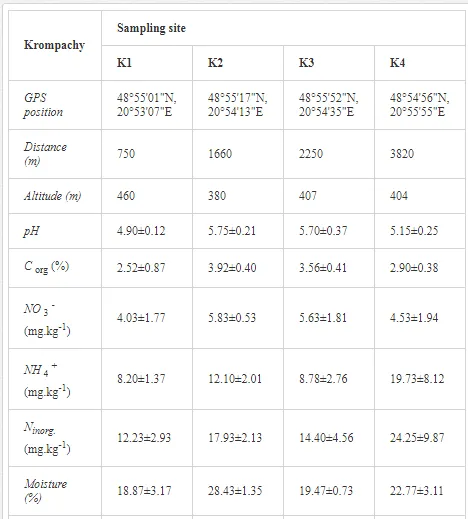
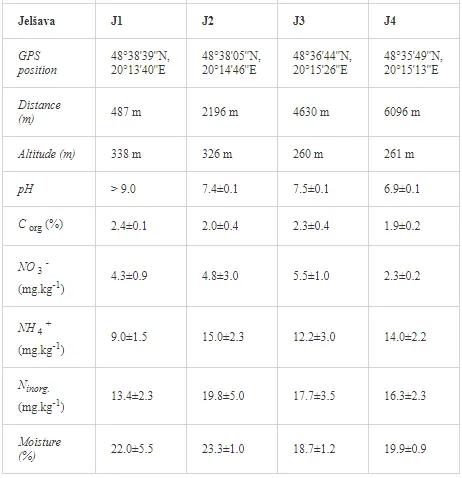
Introduction
Environmental pollution caused by petroleum is of great concern. This is because petroleum hydrocarbons are toxic to all forms of life and harm both aquatic and terrestrial ecosystems. The pollution of marine habitats has caught the attention of researchers and environmentalists. This is due to the serious impact of oil spills on marine life, as well as on people whose career relies on the exploitation of the sea’s resources. Additionally, marine life may be affected by clean-up operations. It may also be indirectly affected by the physical damage to the habitats in which plants and animals live in.Petroleum marine fuel spills, which result from damage, transportation accidents and various other industrial and mining activities, are classified as hazardous waste [1]. They are considered to be the most frequent organic pollutants of aquatic ecosystems [2-3].In recent years, there have been numerous studies regarding the levels of contamination of the seawater by hydrocarbons. The majority of these studies were conducted following the Gulf War of 1991 [4-11] and after, the BP Deepwater Horizon (DWH) oil spill on 20th April, 2010 [12-14]. In the current manuscript, we discuss the effects of petroleum oil spills on marine life. It incorporates different sections that outline the major petroleum oil spills associated with the marine environment, chemical composition of crude oil, toxicity of oil and oil dispersants. We discuss the major petroleum oil spills associated with the chemical and physical states of crude oil and their impact on microbial, plant and animal marine life. We also consider the economic impact of oil spills on coastal activities and on the people who exploit the resources of the sea.
Major oil spills related to marine environments
Saltwater bodies are referred to as ‘marine environments’, with the oceans covering about 70% of the earth. Statistics estimate that 3.2 million tonnes of oil per year are released from all sources into the environment. The majority of this oil is due to general shipping and industrial activities [15]. During the Iran-Iraq war (1980-1988), approximately 2 million barrels of oil were discharged into the Arabian Gulf sea water. These included 1.5 million barrels from the Nawruz blow-out in 1983 [16]. Following the Gulf War in 1991, between 4 and 8 million barrels (1,000 tonnes = 7,500 barrels) were released into the Gulf and the Kuwaiti Desert, making this the largest oil spill in history at that time [17]. When compared to other major spills, the size of this spill attracted global attention. For example, the Amoco Cadiz off the coast of Brittany (France), spilling 200,000 tonnes (1.5 million barrels); the Torrey Canyon, Braer, Sea Empress and the super tanker Breaf off the coast of Shetland (UK) in 1993, spilling a maximum of 84,000 tonnes (607,300 barrels); the Exxon Valdez in Prince William Sound or Alaska (US), which was approximately 36,224 tonnes (261,904 barrels) [18]. Of the recorded oil spills, more than 80% were less than 1,000 tonnes (7,500 barrels) and only 5% were greater than 10,000 tonnes. An accepted average sample size of an oil spill is about 700 tonnes (5,061 barrels) [15]. Previous observations indicated that the number of large spills (>700 tonnes) has decreased significantly over the last 30 years (Table 1)
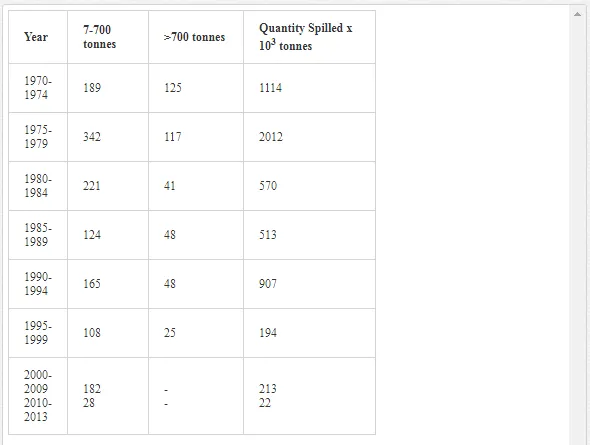

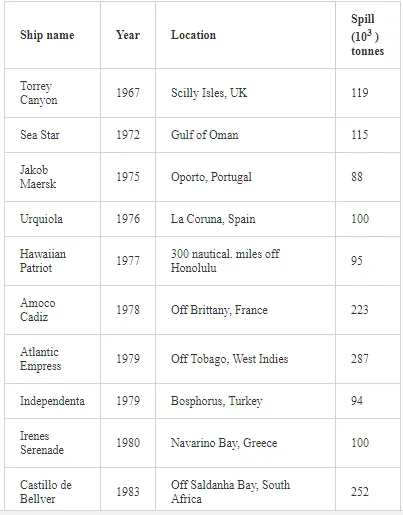
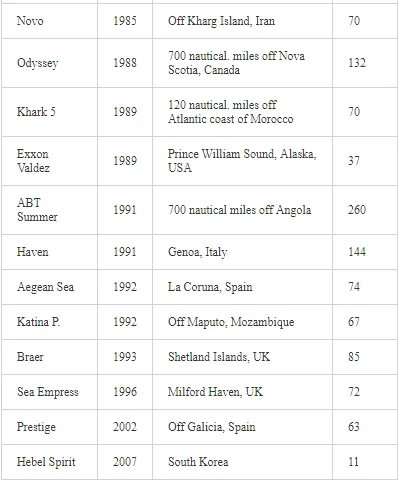
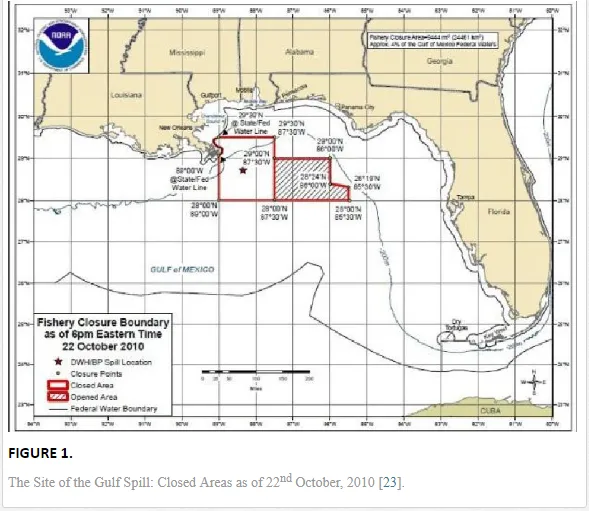
The BP Deepwater Horizon (DWH) oil spill on 20th April, 2010, initiated the discharge of more than 2.6 million gallons (over 800 million litres) of oil into the Gulf of Mexico (Figure 1) over approximately three months. This oil spill was the second largest in human history [19-21]. During the 1991 Gulf War, the deliberate release of over 6 million barrels of oil [22] into the marine environment was considered as the largest in history.
Crude oil and its properties
Crude oil is a complex mixture of organic compounds. These mainly consist of hydrocarbons, in addition to heterocyclic compounds and some heavy metals. The different hydrocarbons that make up crude oil come in a wide range of molecular weights and structure compounds. These compounds include methane gas, high molecular weight tars, asphaltenes, resins, waxes and bitumens. They also include straight and branched chains, single or condensed rings and aromatic rings such as the monocyclic (benzene, toluene, ethylbenzene and xylene). They additionally include polycyclic aromatic hydrocarbons (PAHs) such as naphthalene, anthracene and phenanthrene. Examples of the chemical structure of some common components of crude petroleum are shown in Figure 2.
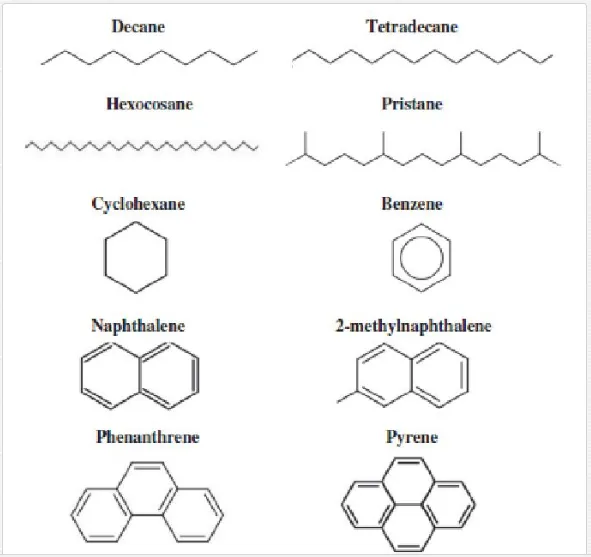
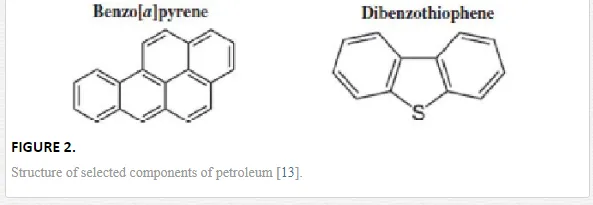
TOXICITY OF OIL
The general effects of oil toxicity depend on a multitude of factors. These include the oil composition and characteristics (physical and chemical), condition (i.e., weathered or not), exposure routes and regimen, and the bioavailability of the oil [24]. One major effect of oil is narcosis, a reversible anaesthetic effect caused by the oil partitioning into the cell membrane and nervous tissue. This causes dysfunctions of the central nervous system [25]. The additive toxic effect of hydrocarbons can lead to mortality, if the levels exceed the threshold concentration [24]. When oil hydrocarbons are ingested by marine animals, they travel to the liver where enzymes activate PAHs to become more toxic and reactive products. The metabolites of polycyclic aromatic hydrocarbons (PAHs) and aliphatic hydrocarbons can be highly toxic and carcinogenic [26]. In particular, PAHs are the major contributors to toxicity, with different metabolic pathways producing metabolites. These have oxidative and carcinogenic properties due to their ability to attack and bind to DNA and proteins [24]. Hydrocarbons have a volatile nature and, therefore, inhalation of them results in respiratory tract irritation and narcosis of mammals and birds. Physical contact is the major route of exposure and usually affects birds and furred mammals. These animals rely on their outer coats for buoyancy and warmth. Consequently, they often succumb to hypothermia, drowning and smothering when oil flattens and adheres to the outer layer [24]. A second general exposure route is through the ingestion or inhalation of the hydrocarbon by organisms that reside on the surface [24]. Exposure by these routes leads to absorption into the bloodstream via the gastrointestinal or respiratory tracts.
TOXICITY OF OIL DISPERSANTS
Oil dispersants (57 chemical ingredients approved for use by the US EPA) are a common tool used after oil spills in marine environments. They break up oil slicks on the water surface and increase the oil’s rate of biodegradation. Oil dispersants are quickly used when other means, such as oil containment and removal, are insufficient. However, consequences of the toxicity of oil spill dispersants alone or in the presence of oil must be evaluated. Generally, undispersed oil poses the greatest threat to shorelines and surface dwelling organisms. However, most dispersed oil remains in the water column where it mainly threatens pelagic and benthic organisms [27]. A complete and updated list of used oil dispersant is available from the US EPA at the website http://www2.epa .gov/emergency-response/alphabetical-list-ncp-product-schedule-products-available-use-during-oil-spill. Several studies have compared the toxicity of oil spill dispersants alone or in the presence of oil. Analyses of tests conducted on a variety of species of aquatic life showed that crustaceans are more sensitive to oil dispersant exposure, compared with fish [28]. A study by [29] indicated that the species with the least amount of protective shell or external tissue is the most sensitive to oil dispersant exposure. It has been shown that the use of oil dispersants increases the exposure and uptake of PAHs by fish. This is particularly the case with fish that live throughout the water column of coastal areas, the ocean and lakes. Researchers found that ‘the risk of PAH toxicity… especially to sensitive life stages, such as eggs and larvae, is enhanced by chemical dispersion’ [30]. In addition, ‘concentrations of LMWPAHs and HMWPAHs (low and high molecular weight PAHs) were found to be higher in the water column following the application of chemical dispersants to the surface slicks’ [31]. ‘For example, see [31]’. They reported that chemical dispersants mobilize PAHs to toxic concentrations as the biomarker ethoxyresorufin-O-deethylase (EROD) activity is increased after exposing newly-hatched mummichog (Fundulus heteroclitus) for 96 h to crude oil and chemically dispersed crude oil.Reference [29] compared the toxicity of a new dispersant, Superdispersant-25 (SD-25), to Corexit 9527 using four species of marine invertebrates at 15 C. The most sensitive species was the snakelocks anemone Anemonia viridis with a 48-hour LOEC of 20 ppm (nominal). This was followed by mussel (Mytilus edulis) feeding rate (50 ppm), seagrass (Zostera marina) photo synthetic index (80 ppm), burrowing amphipod (Corophium volutator) (mortality 175 ppm) and mussel lethality (250 ppm).
Moreover, in a study by [32], adults of four species of wild-caught Newfoundland nearshore fishes were exposed for four days in flow-thru conditions to the dispersant Corexit 9527 alone, water accommodated fraction (WAF) of Hibernia light crude oil alone and dispersed Hibernia crude oil. Test toxicants (20 to 50 ml) were added daily to 300 L tanks for four days, followed by up to six weeks in clean water. The investigators did not report exposure temperatures or toxicant concentrations except to note that initial daily concentrations were 50-100 ppb for Hibernia water-accommodated fraction (WAF). On the first day, the caplein responded to the dispersant by swimming erratically. On the second and subsequent days, they responded by death accompanied by hemorrhaging of the gill lamellae.
Fate of oil spills in the marine habitats
After oil is spilled at sea and with the effect of wind and water current, the oil spreads out and moves on the water surface as a slick a few millimetres thick. At the same time, it undergoes a series of chemical and physical changes [13]. These processes are collectively termed ‘weathering’. Weathering causes the spilled oil to break down and become heavier than water. Some of these processes, like the natural dispersion of oil into water, lead to the removal of the oil from the sea surface and facilitate its natural breakdown in the marine environment. Others, particularly the formation of water-in-oil emulsions, cause the oil to become more persistent and remain at sea or on the shoreline for prolonged periods of time. The speed and relative importance of these processes depend on a number of factors. These include the quantity spilled, the oil’s initial physical and chemical characteristics, weather and sea conditions and whether the oil remains at sea or is washed ashore. Ultimately, the marine environment usually eliminates spilled oil through the long-term process of biodegradation. (http://www.itopf .com/knowledge-resources/data-statistics/statistics/). The natural actions, which are always at work in aquatic environment, are summarized in the US EPA archive document (http://www.epa.gov/oem/docs/oil/edu/oilspill_book/chap1.pdf). These include weathering, evaporation, oxidation and biodegradation.
IMPACT OF OIL SPILLS ON MARINE ORGANISMS
Ultimately, the impact of oil on marine organisms depends on the fate of the oil. As previously described, when oil is present in the environment, it is either dispersed in the top layer of the water (littoral zone) or remains on the surface and, consequently, on the coastal areas. If the oil is not dispersed, it remains on the surface. In this case, currents bring the oil towards coastal areas which harms coastal organisms like invertebrates, mammals and birds. However, if the oil is dispersed, organisms, such as fish, plankton and larvae, are immediately subjected to oil toxicity.
IMPACT OF OIL SPILLS ON PLANKTONIC ORGANISMS
Zooplankton is a particularly important food resource, especially for baleen whales. It can influence or control the primary productivity by top-down effects [33] in return. Its population dynamic change can influence the biomass of other marine animals like fish by bottom-up effects [34]. Some zooplankton, such as copepods, euphausiids and mysids, assimilate hydrocarbons directly from seawater and by ingesting oil droplets and oil contaminated food [35 as cited by 36]. The ingestion of oil by these organisms often causes mortality, while surviving organisms often show developmental and reproductive abnormalities [37].
The impact of petroleum pollution on marine plankton has been a great cause for concern. Reference [37] summarized the reports regarding the toxic effects of oil water accommodated fraction (WAF) on marine phytoplankton, zooplankton and the early life stages of animals. Generally, oil WAF toxicity enhances with increasing carbonic chain length and benzene ring number. The paper summarized the research results regarding the influence of oil WAF on marine plankton. It also suggested future study points to further promote the quantified evaluation of the damage by oil pollution to marine ecology. For the oil WAF, [37] reported that plankton are capable of accumulating PAH due to their great lipophilic abilities. They, therefore, stimulate various harmful effects. The investigators reported that marine plankton is highly sensitive to the petroleum WAF, as the order of median effective/lethal concentration is as low as lg/L or mg/L. Examinations of the toxicity effect of 10 polycyclic aromatic hydrocarbons associated with the Prestige fuel oil spill on adult copepods (Oithona davisae) revealed that the PAHs had narcotic effects on these organisms [38].
IMPACT OF OIL SPILLS ON BENTHIC ORGANISMS AND INVERTEBRATES
Benthic invertebrates and higher forms, such as the sand eel and Ammodvtes americanus (a main food resource of Atlantic humpback whales) [39 as cited by 36], may accumulate petroleum hydrocarbons from water, contaminated sediments and food [40 as cited by 36]. Thus, these whales are adversely affected.
Bivalve molluscs tend to accumulate petroleum hydrocarbons to higher concentrations and retain them longer than other taxa [41-42 as cited by 36]. This is essentially due to the lack of a mixed function oxygenase (MFO) system [43 as cited by 36] that makes them unable to metabolize the compounds to execrable polar metabolites. Thus, they are likely to transfer them to their predators. Marine mammals that rely heavily on bivalve molluscs for food, such as the walrus and otter, share a higher risk of ingesting petroleum hydrocarbons [44 as cited by 36]. Benthic amphipods are quite sensitive to spilled oil. They are among the first marine animals killed and the slowest to recover [45 as cited by 36]. However, most marine crustaceans have a well-developed MFO system [43 as cited by 36]. As a result, they are able to metabolize and excrete accumulated hydrocarbons quite rapidly. Previous studies have explored the recovery of the invertebrate populations after oil spills [46-47]. In the intertidal habitat, the biological recovery of the exposed shores is faster than the sheltered shores. This is because strong wave action promotes the removal of contamination and the animals and plants of exposed shores tend to be severely affected. Thus, they are better able to re-colonize an impacted shore quickly. Sublittoral habitats are generally contaminated by sedimentation of oiled particulate material and clean-up for these habitats is not practiced. As a result, the recovery of subtidal communities impacted by oil spills usually takes a longer time. For example, the Abra alba bivalve sand community in the Bay of Morlaix, Brittany was severely affected by the Amoco Cadiz oil spill (1978) [47]. After the spill in 1978, the biomass values for the sand community immediately fell. However, within two years, they recovered to pre-spill levels. Productivity also showed similar trends.
Invertebrate populations, such as the amphipod sand hopper, Ampelisca, are quite sensitive to oil pollution and, for various reasons, are slow to re-populate and ‘recover’. For example, the initial impact of the spill in the Bay of Morlaix, following the Amoco Cadiz spill in 1978, was to kill off populations of the amphipod sand hopper, Ampelisca, which dominated the community [46]. Although the sediment was rapidly purged of the contaminating oil, it has been noted that Ampelisca was back to its pre-spill population density after 10 years. The standing crop biomass and productivity was restored much more rapidly as the ecological niches had been occupied by other opportunists like the bivalve Abra and the worm Nephtys. These had quickly filled the place left by the Ampelisca [46].
In other studies on oil spill accidents, it has been shown that six months after the Prestige oil spill and clean-up campaign, invertebrate populations of the exposed sandy beaches, notably the isopod Eurydice, the spionid polychaete Scoleleoius squamata, nemerteans and Diptera, were significantly reduced. Furthermore, their abundance inversely related with the oil pollution gradient. The number of taxa was reduced but not the diversity values. The only clam on these sandy beaches was Donax. Before the spill and clean-up, it occurred in six beaches but afterwards, only in one. Upper dry sand communities were particularly reduced due to both oil toxicity and extensive beach grooming that also removed seaweed wrack [48-49]. Marine invertebrates are unique living organisms in different aspects. They have been explored as a model for several biological markers as a result of oil spill pollution. Reference [50] surveyed the marine invertebrate mussels (Mytilus edulis) exposed in situ to the oil that came ashore after the wreck of the ‘Erika’ tanker on the Brittany (France) coast in December, 1999. The mussel response was assessed using a set of biomarkers (acetylcholinesterase (AChE), glutathione S-transferase (GST), catalase (CAT), malondialdehyde (MDA) and deoxyribonucleic acid (DNA) adducts, related to the metabolism of the organic contaminants. The results of a series of validation tests revealed that there were no significant reductions in the GST or CAT levels. Six months immediately following the accident, observations indicated that the DNA adducts and MDA levels were high and the levels of AChE were significantly lower during the first year of the survey. This suggested a general stress. A simple multivariate graphic method – the integrated biomarker response index – was used to combine four of the five validated biomarkers and quantify the degree of impact on mussels at different sites. The results indicated that mussel populations were affected by the oil spill during only the first year after the accident.
IMPACT OF OIL SPILLS ON CORAL REEFS
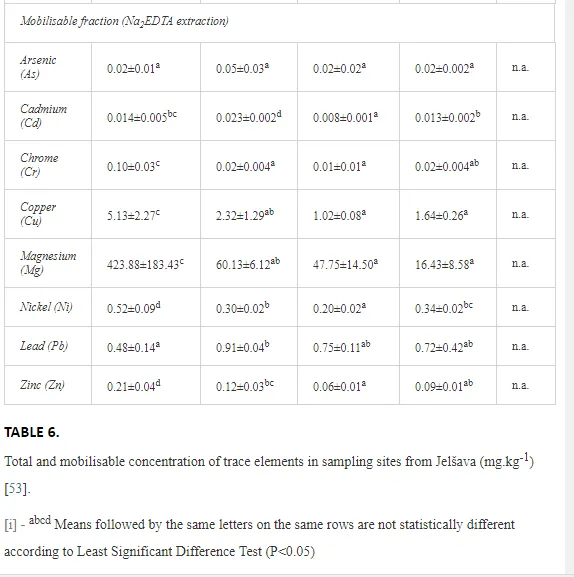
In addition, recreational attractions for divers, coral reefs are considered to be important constituents of marine ecosystems. This is because they are important nurseries for shrimp, fish and other animals [51]. The aquatic organisms that live within and around the coral reefs are at risk of exposure to the toxic substances within oil, as well as smothering. They are rapidly deteriorating because of a variety of environmental and anthropogenic pressures. Thus, they are suffering significant changes in diversity, species abundance and habitat structure worldwide [52]. Oil dispersants are potentially harmful to marine life including coral reefs [53]. In a study using coral nubbins in coral reef ecotoxicology testing, [54] found that dispersed oil and oil dispersants are harmful to soft and hard coral species at early life stages. The investigators also employed a ‘nubbin assay’ on more than 10, 000 coral fragments to evaluate the short- and long-term impacts of dispersed oil fractions (DOFs) from six commercial dispersants (Slickgone, Petrotech, Inipol, Biorieco, Emulgal and Dispolen) and the dispersants and water-soluble-fractions (WSFs) of Egyptian crude oil on two Indo-Pacific branching coral species, Stylophora pistillata and Pocillopora damicornis [53]. They found that the dispersant concentrations recommended by the manufacturer were highly toxic and resulted in mortality of all nubbins. The dispersed oil and the dispersants were significantly more toxic than the crude oil WSFs. As corals are very sensitive to oil detergents and dispersed oil, the results of these assays indicated the negative and harmful effect of using any oil dispersant in coral reefs and in the area closely around them. These dispersants were rated based on their eco-toxicological impacts on the corals. Observations indicated a scale from the least to the most harmful agent, as follows: Slickgone > Petrotech > Inipol > Biorieco > Emulgal > Dispolen.
IMPACT OF OIL SPILLS ON FISH
Due to the well-developed hepatic mixed function oxygenase (MFO) system, in addition to the reactivity of the metabolites that would not be released in a toxic form during digestion and absorption, most fish, even in heavily oil-contaminated environments, do not accumulate and retain high concentrations of petroleum hydrocarbons. Thus, they are not likely to transfer them to predators. Thus, no serious threat is predicted [55 as cited by 36]. Generally, marine carnivores are inefficient assimilators of petroleum compounds in food. For this reason, and because all prey species are able to release hydrocarbons from their tissues [41 as cited by 36], the marine food chain biomagnification does not occur. Thus, there is an indirect correlation between a marine mammal’s trophic level and the concentration of residues that it might consume. In fact, as top carnivores that feed on large pelagic fish and seals, polar bears and killer whales are less likely to be exposed to petroleum in their food than other species, such as walrus and baleen whales, which feed on zooplankton and benthic invertebrates.
Experiments that explain the effect of oil spills on the early stages of fish were demonstrated by [56]. They found that chronic exposure of juvenile pink salmon (Oncorhynchus gorbuscha) to Alaska North slope crude oil resulted in a variety of responses. These included melanosis, erratic swimming, loss of equilibrium, reduced mobility and startled response. Interestingly, the effects were not enhanced by the phototoxicity from UV irradiation, presumably of the highly pigmented nature of the fish’s skin (in contrast to phototoxicity in translucent early life stages of marine invertebrates). When they examined the phototoxicity of the water accommodated fractions of weathered crude oil, they found that pink salmon may be at less risk of photoenhanced toxicity, compared to the more translucent early-life stages of several other Alaskan species.
IMPACT OF OIL SPILLS ON SEABIRDS
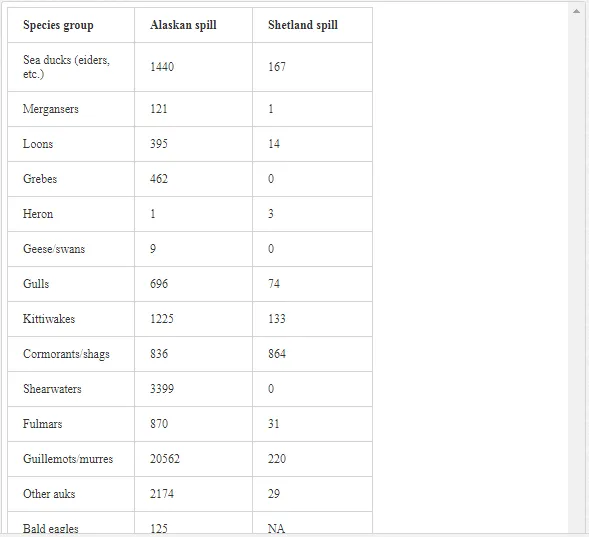
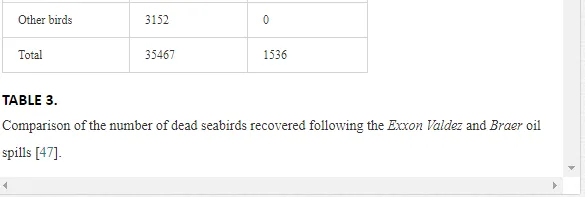
As one of the major routes of exposure, physical contact usually affects birds. For example, thousands of African penguins (Spheniscus demerus) were oiled following the 2000 Treasure oil spill in South Africa. An evaluation of the impact of oil spills on seabirds has not been fully appreciated during incidents, despite pressure from the public concern, media and other interested parties for precise and up-to-date information on the damage. Consequently, the approximate numbers of seabird casualties involved in many major spills have only been estimated, while impacts at the population level have been difficult to determine. Natural variation and the huge range of factors that influence bird population statistics make it difficult to assess the impact of oil spill on sea birds. In reference [57], in their efforts to assess the impact of oil spills on seabirds in Europe and in North America, the investigators reported that there are two inter-linked aspects to dealing with oiled seabirds during major spills. The first is a relatively expensive and logistically complicated process. This involves birds found alive and the humanitarian efforts made to clean, rehabilitate and successfully release them into the wild. The second involves assessing the likely impact of the spill on the populations of those species affected. The relation between the size of an oil spill and the number of seabird casualties is not directly proportional. Moreover, estimates of the number of seabird deaths from oil slicks are highly speculative. This is because an unknown number of oiled birds may die at sea and not reach the coast. For example, following the Exxon Valdez oil spill, over 35,000 seabird carcasses were recovered in the northern Gulf of Alaska [58]. However, after the Braer accident [59], even though the Braer spill (85,000 tonnes) was almost two and a half times as large as that of the Exxon Valdez (Table 3), only 1,500 dead birds were counted.
Researchers argued that the rapid recovery of the murre breeding colonies in Alaska and the number of dead birds might be lower than was estimated. They also suggested that breeding pairs may have been replaced by younger birds that came in from the open sea [60]. On the other hand, [61] argued that the impacts of the spill may have been diffused over a large area, permitting local recovery and making it difficult to detect any changes in local abundance or habitat occupancy. Nine years after the Exxon Valdez oil spill, in their long-term effects observation, [62] claimed that the populations of most bird species have not recovered and others still show potential population effects. However, the report is at variance with other findings [63-64]. During the winter season of 2003, approximately 64,000 tonnes of Prestige heavy fuel oil spilled off Galacia, Spain. As a result, 10 % of the European shag (cormorants, Phalacrocorax aristotellid) were killed. In turn, this resulted in a 50% reduction in the 2003 breeding success of oiled colonies, compared to the unoiled colonies [65]. Reference [66] found that the kidneys of 32 oiled shag had extensive tissue damage and haemorrhaging. They further found that the oiled shag were heavily infected with an eimeriorin Apicomplex coccidian, which under normal circumstances is not pathogenic
IMPACT OF OIL SPILLS ON MARINE MAMMALS
Marine mammals include bottlenose dolphins, fins, humpbacks, rights, sei whales, sperm whales, manatees, cetaceans, seals, sea otters and pinnipeds. As previously indicated, the physical contact of oil with furred mammals usually affects these animals because they rely on their outer coats for buoyancy and warmth. Consequently, these animals often succumb to hypothermia, drowning and smothering when oil flattens and adheres to the outer layer [24].
As part of their activities, all marine mammals spend a considerable amount of time at the surface. Here, they swim, breathe, feed or rest. Thus, the possibility of their contact with a surface slick, water-in-oil emulsion, or tar balls, is high. In heavy pelage marine mammals, such as fur seals, sea otters and polar bears, this contact may lead to fouling. Polar bears and otters groom themselves regularly as a means of maintaining the insulating properties of the fur and may, thereby, ingest oil. Animals with smooth surfaces or relatively little to no pelage, such as whales, dolphins, manatees and most seals, have an advantage as oil would have fewer tendencies to adhere to their surface [36]. Some baleen whales are skim-feeders, i.e., they eat at the surface [67 as cited by 36]. When in an area of slick or tar balls, this behaviour can lead to foul in the feeding apparatus. Tarry residues, in particular, can coat the baleen plates. Animals, such as narwhals, belugas, ringed seals, walruses and polar bears in Polar Regions, spend most of their time at the ice edge in leads, polynyas and breathing holes. This is where spilled oil tends to accumulate. Oil that contaminates a shore is likely to severely affect pinnipeds. Pinnipeds require such areas for nursery and, to a lesser extent, otters and bears. Some of the oil is eventually returned in subtidal sediments, where it may transfer to gray whales, walrus and some seals. Such species feed heavily on benthic animals. When marine mammals encounter fresh oil, they are likely to inhale volatile hydrocarbons evaporating from the surface slick. These volatile fractions contain toxic monoaromatic hydrocarbons (benzene, toluene and xylenes) and low molecular weight aliphatics with anaesthetic properties. The inhalation of these volatile hydrocarbon compounds is potentially harmful [68-69 as cited by 36]. The inhalation of concentrated petroleum vapours can cause the inflammation of and damage to the mucus membranes of airways, lung congestion or even pneumonia [70 as cited by 36]. Volatile benzene and toluene, which can be inhaled, can be transferred rapidly from the bloodstream into the lungs. Furthermore, they can accumulate from the blood into the brain and liver, causing neurological disorders and liver damage [71 as cited by Neff 36]. Marine mammals are probably poor accumulators to oil directly from the solution or dispersion in the water column. This is because the skin of cetaceans is relatively impermeable to oil [72 as cited by 36]. Additionally, most marine mammals do not drink large volumes of seawater. Thus, a significant accumulation of hydrocarbons by this route is unlikely to occur.
There is an extensive and diverse database regarding subjects that deal with effects of oil on marine mammals and those aspects of an animal’s life history vulnerable to exposure of spilled oil. This database is summarized in a publication by the Department of Interior/Minerals Management Service (MMS 88-0049)/Atlantic OCS region, Canada [72]. ‘For example, see [36]’, described the effects of oil on marine mammal populations. At the same time, [73] described the physiological and toxicological effects of oil on each of the marine mammal groups. Reference [73], in his study of ‘Physiologic and Toxicologic Effects on Pinnipeds’, indicated that pinnipeds are inappropriately sensitive to the harmful properties of oil. Incidental ingestion during feeding, exposure to vapour concentrations and surface contamination with relatively fresh oil does not appear to cause a disaster. However, Pinnipeds trapped near the source of a spill or those which are forced to emerge in heavy accumulations of oil in leads and around rookeries exhibit the most severe effects. Experimental studies by the same investigators on fur seals indicated that surface fouling decreases the insulation value of the pelt. This can potentially lead to thermal and energetic stress. Furthermore, the sensitivity to the effects of oil exposure may be high in species and groups that are compromised by pre-existing disease, or stressed by pressures of an unfavourable habitat, intra-specific competition or unusual environmental conditions. [73].
IMPACT OF OIL SPILLS ON MARINE PLANTS
In several aspects, aquatic plants are important to the functioning of ecosystems. These include the fact that they are oxygen producers, their ability to sequester carbon and for their base position in aquatic food chains. In addition, they serve as nursery, feeding and breeding habitats for a variety of animal and plant species, including recreationally and commercially important fish. Plants and animals are affected by the oil in which they come into contact with as a result of an oil spill. In their review of toxicities of oils, dispersants and dispersed oils to algae and aquatic plants, [74] summarized the reported phytotoxicities of oils, dispersants and their combinations to aquatic plants. They assessed the ability of the reviewed database to support toxicity predictions and evidence-based risk assessments. The phytotoxicity database mainly includes the results of research conducted after oil spills to marine waters. The toxicity of at least 41 crude oils and 56 dispersants were recorded. At least 107 response parameters were monitored for 85 species of unicellular and multicellular algae, 28 wetland plants, 13 mangroves and nine seagrasses. Due to experimental diversity, the effect concentrations available from this toxicity database are varied and diverse. As a result, there are restricted phytotoxicity predictions and identification of sensitive species, life stages and response parameters. Thus, the impact of toxicity of petrochemicals and dispersants on aquatic plants was not supported by this database.
Reference [74] recommended a proactive and experimentally-consistent approach to provide the threshold toxic effect concentrations for sensitive life stages of aquatic plants inhabiting diverse ecosystems. The effects of heavy fuel oil contamination on the growth and the development of Salicornia fragilisBall and Tutin, a salt-marsh edible species, were studied as greenhouse experiments by [75]. Phytotoxicity assessments and PAH shoots assays were followed to measure the impact of petroleum on plant development. Syptoms like chlorosis, yellowing, growth reduction and perturbations in developmental parameters were visually observed. In this study, shoot coating appeared to be less than through soil and the investigators observed more marked effects on plants as an indication of the degree of pollution. However, a significant bioaccumulation of PAHs in shoot tissues was also found, even at very low levels of contamination. These highly related to the conditions of exposure to oil. This strong relationship between the PAH contents of Salicornia plants and growth reduction suggest a chemical toxicity of fuel oil. The investigators concluded that the type and degree of fuel oil contamination are two important factors in controlling the impact of fuel oil on S. fragilis.
In 1986, more than 8 million litres of crude oil spilled into a complex region of mangroves, seagrasses and coral reefs, just east of the Caribbean entrance to the Panama Canal [76]. Intertidal mangroves, sea grasses, algae and associated invertebrates were covered by oil and died soon after. Investigators reported that seedlings of red mangrove, Rhizophoram angle, which were transplanted to heavily oiled sites, did not produce new leaves. This contrasted the transplants at an unoiled site. Entire beds of intertidal seagrass Thalassiat estudinum were killed on some heavily oiled reef flats, as shown by the abundant oil-covered dead leaves washed ashore, as well as the dead, but intact, root-rhizome mats. In contrast, the subtidal Thalassia survived everywhere after the spill. Having said this, in the heavily oiled areas, the leaves of the subtidal Thalassia became brown and heavily fouled by algae for several months. Another example of the oil spill effect on mangrove plants is December, 2000. In this case, 500 mangrove saplings in a 6.34 ha reserve in Hong Kong were subjected to a smuggled fuel oil spill. More than 80% died in root-zone sediments containing 60 – 80 ug/g total petroleum hydrocarbons (TPH). However, one year later (December, 2001), the injured survivors had recovered and re-grown, with root-zone sediment TPH concentrations approaching urban ‘background’ values of 40 ug/g TPH [77].
IMPACT OF OIL SPILLS ON CYANOBACTERIA AND OTHER MICROORGANISMS
There are numerous factors that determine the microbial response to marine oil spills. These include the oil composition and degree of weathering, as well as the environmental conditions, particularly temperature and nutrient concentrations. Reference [13, 78-80], for example, reviewed the different factors affecting the biodegradation of the petroleum hydrocarbons by microorganisms and how environmental and biological factors could determine both the rate at which and extent to which hydrocarbons are removed from the environment.
When crude oil is introduced into seawater, the microbial community changes and consists of multiple co-existing species. These can be explained by resource sharing [81]. In reference [13], in their review paper, reported that the diverse array of hydrocarbons present in crude oil requires resource partitioning by microbial populations, as well as microbial modification of oil components and the surrounding environment, will lead to temporal succession. The reviewers observed a network of direct and indirect interactions within and between species, even when just one type of hydrocarbon is present. They also provided a schematic illustration (Figure 3) of some of the interactions involved in hydrocarbon biodegradation. Elements of these interactions were stated in several studies reviewed by them.
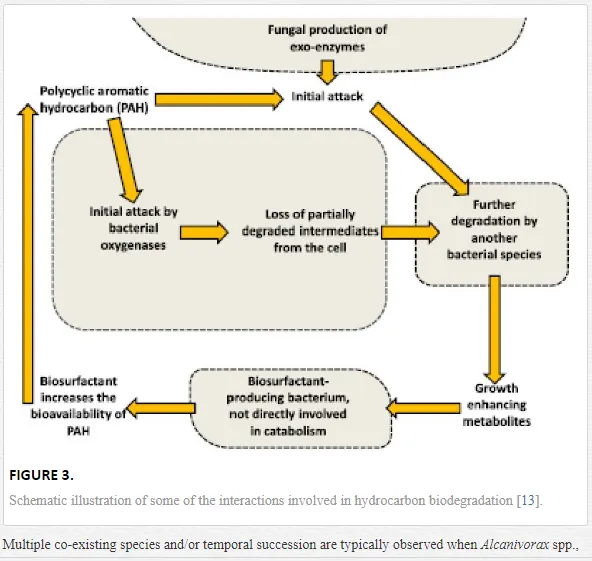
Multiple co-existing species and/or temporal succession are typically observed when Alcanivorax spp., which degrade straight-chain and branched alkane, are increased and found abundantly [82-88]. This is followed by Cycloclasticus spp., which degrade PAHs [82-86, 89-92]. Other genera of obligate alkane degraders, such as Oleibacter sp. [87, 93] and Oceanospirillales sp. [94], have been abundantly detected in other oil-rich marine environments [95-96].
At the molecular level, studies by the microarray analysis of 16S rRNA genes have revealed that Gamma-proteobacteria were enriched in marine environments that are historically exposed to frequent, episodic, natural ‘oil spills’ [97]. This analysis also revealed that marine water exposed to oil spills enriches indigenous oil degrading bacteria, where members of the order Oceanospirillales comprised more than 90% of the bacterial community, compared to 5% of the uncontaminated sample [97]. Experiments on the incubation and mixing of marine sediment with phenanthrene and bromodeoxyuridine (BDU), followed by the analysis of BDU-labelled DNA, revealed diverse groups of PAH degraders belonging to the genera Shewanella, Exiguobacterium, Methylomonas,
Pseudomonasand Bacteroides. It also revealed Gammaproteobacteria and Deltaproteobacteria, which were not closely related to the cultivated organisms [98].
Following the 1991 Gulf War environmental disaster, an extensive formation of cyanobacterial mats was observed to colonize most of the oil polluted shores.
Although most of the intertidal cyanobacterial mats were severely affected by the oil spill, these organisms were the first to re-colonize the destroyed habitats [99]. This initial massive growth of cyanobacteria, especially on sites where they did not occur before the oil spill, indicated the preference of the cyanobacterial mats to the absence of bioturbation (i.e., destabilizing the sediment surface caused by crabs and polychaetes) of the sediment for their growth. Cyanobacteria do not usually occur where bioturbation has been carried out and – together with the grazing pressure by benthic animals – prevents the establishment of cyanobacterial mats. The extensive growth of cyanobacteria following the oil pollution of the shores can be explained by destroying most of the crab colonies in the mudflats and immediately stopping the bioturbation process, as well as grazing by gastropods. Reference [100] explained this colonization by three different processes. The first one is the desiccation, cracking and peeling of the cyanobacterial mats. This removes the uppermost part of the oiled sediment. The second is the resettlement of burrowing macrofauna like crabs and benthic animals, such as gastropods, which outcompete the cyanobacteria again. The third is further extensive growth of cyanobacteria building thick laminated mats. These layers completely seal the surface and hence, produce an anaerobic zone which inhibits oil degradation. As long as such a bloom of cyanobacteria exists, microbial oil degradation will be prevented. They will also prevent any resettlement by macrofauna.
Communities at risk of marine oil spills/anticipation and preparation
The threatening of marine environments with the petroleum oil spills has caught the attention of many communities, encouraging them to develop their own plans and policy issues. These have ranged from permitting or prohibiting increased oil transport volumes, to developing the capacity to respond to and recover from potential spill disasters. A comprehensive literature review was conducted by [101], who covered 300 academic, governments and industry papers and reports relating to oil spills and their environmental and societal consequences, with emphasis on economic impacts. Reference [14], in their report of the ‘Consequences of Oil Spills from Tanker Accidents’, provided a review and structured framework that supports the efforts of such communities to anticipate the spectrum of issues, factors, stakeholders and strategies that may be involved. The review presented an initial and important input into the larger process of addressing the risk of oil spill disasters. The research was based on two premises. The first was that, although previous disasters provide an essential information source for anticipating future events, not all lessons may be transferrable across locales and the key to successful planning and learning from experience is that it be based on systematic assessment activities’ [102]. The second premise was that developing realistic expectations of oil spill consequences requires an understanding of the full range of impacts and interactions within and across the affected systems. These include the marine ecosystems and socioeconomic systems.
The purpose of the summary literature review and overview framework provided by [14] was to help communities systematically consider the factors and linkages that would influence consequences of a potential oil spill. Studying previous oil spill disasters has assisted communities to focus on several main domains of interest. These include the nature of the oil spill itself, how to manage the disaster, the physical marine environment, marine biology, human health, economy and policy. Key factors that influence the severity of the impact were identified and significant interactions between variables were recognized. By using this framework, it is suggested that communities can clarify the complexity of oil spill impacts, develop experience for planning from other oil spill disasters and develop the capacity to respond to and recover from potential spill disasters. Furthermore, such a framework encourages debates about risk analysis and policy to understand and reduce the susceptibility of their localities to potential spill disasters. The investigators concluded that a comprehensive overview can help clarify the complexity of oil spill disasters, make comparisons across events, identify data gaps and develop planning scenarios in preparation for future oil spill disasters. Local communities that depend on the fishing industry, aquaculture and tourism should realize that the impact of an oil spill is governed by complex factors. These include the oil spill’s volume and location relative to fishing/cultivation areas, currents, tides and wave action. Other factors include whether species harvested in the region are sedentary or mobile, as well as government decisions relating to fishing bans and compensation schemes.
Economic impact of oil spills in the Gulf
During the 1930s and 1940s, the discovery of oil in the Gulf led to a massive increase in shipping. This discovery is principally responsible for the huge economic wealth and strategic importance associated with the region today. Thus, the socio-economic development of the Gulf region is highly dependent on its marine environmental quality. In 1991, the second Gulf War led to the largest oil spill in human history. Around 6 million barrels of oil were discharged into the Gulf [22]. Moreover, 770 km of coastline from southern Kuwait to Abu Ali Island (Saudi Arabia) were smothered with oil and tar, erasing most of the local plant and animal communities. Many of these communities were internationally significant. For example, a number of bird species, green and hawksbill turtles and dugongs are endemic to the region. Such species were harmed and disturbed [100].
Considering that approximately 49% of the world’s oil production comes from the Gulf States and passes through the Gulf, its liability to pollution is about 48 times that of any other similar area on earth [103]. Hence, the Gulf is possibly the most chronically oil-polluted marine area in the world, even before the war [104]. Due to the different anthropogenic activities relating to oil spills, in addition to the natural environmental stresses of the Gulf, i.e., enclosed and shallow nature, a number of socio-economic impacts are predicted:
a. Fisheries as a multi-million dollar industry and the artisanal fisheries as a resource of great social significance are threatened. This is because oil spills are harmful to coral reefs, mangrove areas and seagrass. These organisms provide a support and grounds for a number of commercially significant fish and shrimp species.
b. Desalination plants that provide most of the population’s freshwater supply for the Gulf region are threatened.
c. Disruption to the fishing industry and a reduction in scuba diving tourism [105]. Consequently, people who have a career in this industry will lose their jobs, which they have had for a long time.
d. Disruption of the coastal and marine environments which are internationally significant for a number of bird species, green and hawksbill turtles and dugongs. These are endemic to the region.
Conclusion
Marine oil spills can have a serious impact on marine life, as well as on the economic coastal activities and the communities that exploit the resources of the sea. Generally, the effects of oil toxicity depend on a multitude of factors, including the oil composition and characteristics (physical and chemical), condition (i.e., weathered or not), exposure routes and regimen, and bioavailability of the oil. Oil dispersants, which are a common tool used after oil spills, are also toxic and threaten pelagic and benthic organisms, as well as fish. Marine life can also be affected by clean-up operations or indirectly through the physical damage to the habitats in which plants and animals live. Communities that are threatened by marine oil spills have realized the risk and have, therefore, developed their own plans and policy issues to counteract the risk of marine oil contamination. Due to the different anthropogenic activities relating to oil spills, in addition to the natural environmental stresses of the Gulf, a number of socio-economic impacts are predicted. These are summarized by the threatening of the fish industry and desalination plants that supply most of the populations’ freshwater for the Gulf region, in addition to the scuba diving tourism.