By moses dhilip kumar
abstract
The
hydrogen gas injector plug for generating hydrogen gas from ambient air drawn
into the piston chamber of an engine includes an array of nozzles fabricated
from a metal or a mixture of metals. When heated to a pre-determined
temperature, the metallic nozzles react with atmospheric water to disassociate
hydrogen gas. The hydrogen gas is injected from the nozzles into the piston
chamber and mixed with air. An ignition device ignites the mixture of hydrogen
and air so that it burns to power the piston in a conventional manner. The
metallic nozzle is heated via a copper conductor connected to a source of
electric current.
NEED FOR ALTERNATIVE FUELS
The
rising cost and diminishing supply of hydrocarbon fuels, i.e. gasoline, have
increased the criticality of developing or finding alternative fuels.
Furthermore, pollution caused by burning hydrocarbon fuels is suspected of
creating a “greenhouse” effect in the atmosphere, thereby creating problems
that may have a bearing on the future course of human civilization. The world
would certainly welcome a device that could utilize a virtually inexhaustible
supply of a common element to power internal combustion engines, which device
would also cause production of pollution-free byproducts. Some well known
alternative fuels include biodiesel, bio-alcohol (methanol, ethanol, butanol),
chemically stored electricity (batteries and fuel cells), hydrogen, non-fossil
methane, non-fossil natural gas, vegetable oil and other biomass sources.
HYDROGEN GAS AS AN ALTERNATE FUEL
Hydrogen
is one of two natural elements that combine to make water. Hydrogen is not an
energy source, but an energy carrier because it takes a great deal of energy to
extract it from water. It is useful as a compact energy source in fuel cells.
Many companies are working hard to develop technologies that can efficiently
exploit the potential of hydrogen energy. The use of hydrogen as a fuel to
power engines has been contemplated for many years. Combustion of this hydrogen
produces only pollution-free water. Unfortunately, hydrogen poses many risks
when stored in large quantities, thus creating many problems in making the gas
available to the general public. Thus a hydrogen gas injector plug solving the
aforementioned problem is desired.
Hydrogen does not come as a
pre-existing source of energy like fossil fuels, but is first produced and then
stored as a carrier, much like a battery. Hydrogen for vehicle uses needs to be
produced using either renewable or non-renewable energy sources. A suggested
benefit of large-scale deployment of hydrogen vehicles is that it could lead to
decreased emissions of greenhouse gases and ozone precursors.
A hydrogen vehicle uses hydrogen as
its onboard fuel for motive power. The term may refer to a personal
transportation vehicle, such as an automobile, or any other vehicle that
uses hydrogen in a similar fashion, such as an aircraft. There are basically
two conventional methods of powering a engine using hydrogen, they are:
·
In hydrogen internal
combustion engine vehicles, the hydrogen is combusted in engines
in fundamentally the same method as traditional internal combustion engine vehicles.
- In
fuel-cell conversion, the hydrogen is reacted with oxygen to produce water
and electricity, the latter being used to power an electric traction
motor.
•
Wide range of flammability
•
Low ignition energy
•
Small quenching distance
•
High auto-ignition temperature
•
High flame speed at stoichiometric ratio
•
High diffusivity
•
Very low density
But...
The
challenges in using the hydrogen in vehicles are production, storage, transport
and distribution. Each of these requires great amounts of energy and safety
precautions. Because of all these challenges the efficiency of hydrogen will
not exceed 25%.
HYDROGEN
PRODUCTION
The
molecular hydrogen needed as an on-board fuel for hydrogen vehicles can be
obtained through many thermo-chemical methods utilizing natural gas, coal (by a
process known as coal gasification), liquefied petroleum gas, biomass (biomass
gasification), by a process called thermolysis, or as a microbial waste product
called bio-hydrogen or Biological hydrogen production. Most of today's hydrogen
is produced using fossil energy resources and 85% of hydrogen produced is used
to remove sulfur from gasoline. Hydrogen can also be produced from water by
electrolysis or by chemical reduction using chemical hydrides or aluminum.
Current technologies for manufacturing hydrogen use energy in various forms,
totaling between 25 and 50 percent of the higher heating value of the hydrogen
fuel, used to produce, compress or liquefy, and transmit the hydrogen by
pipeline or truck. Electrolysis is currently the most inefficient method of
producing hydrogen, uses 65 to 112 percent of the higher heating value on a
well-to-tank basis
In
addition to the inherent losses of energy in the conversion of feed stock to
produce hydrogen, which makes hydrogen less advantageous as an energy carrier,
there are economic and energy penalties associated with packaging,
distribution, storage and transfer of hydrogen.
DIFFICULTIES
IN STORING HYDROGEN GAS
Hydrogen
for use as fuel must first be produced using another energy source, making it a
means to transport energy, rather than an energy source, similar to a
rechargeable battery. One existing method of hydrogen production is steam
methane reformation; however, this method requires methane (most commonly
available as natural gas), which raises sustainability concerns. Another method
of hydrogen production is through electrolysis of water, in which electricity
generated
from any
source can be used. Photo electrolysis, bio-hydrogen, and biomass or coal
gasification have also been proposed as means to produce hydrogen.
Hydrogen
is currently impractical as an alternative to fossil-based liquid fuels. While
hydrogen has a very high energy content by weight, it has very low energy
content by volume, making it very challenging to store in average-sized
vehicles. Hydrogen can be stored as compressed hydrogen, as cryogenic liquid
hydrogen, or in compounds containing hydrogen which must undergo a chemical
change to release the gas such as metal hydrides. However, because of the lower
volumetric energy, hydrogen gas tanks would need to be two to three times as
large for compressed hydrogen storage as conventional gasoline tanks.
Although hydrogen fuel
cells generate no CO2, production of the hydrogen for cars currently
creates higher emissions than gasoline cars. While methods of hydrogen
production that do not use fossil fuel would be more sustainable, currently
renewable energy represents only a small percentage of energy generated, and
power produced from renewable sources can be used in electric vehicles and for
non-vehicle applications.
Hydrogen
has a very low volumetric energy density at ambient conditions, equal to about
one-third that of methane. Even when the fuel is stored as liquid hydrogen in a
cryogenic tank or in a compressed hydrogen storage tank, the volumetric energy
density (mega joules per liter) is small relative to that of gasoline. Hydrogen
has a three times higher energy density by mass compared to gasoline (143 MJ/kg
versus 46.9 MJ/kg). Because of the energy required to compress or liquefy the
hydrogen gas, the supply chain for hydrogen has lower well-to-wheel efficiency
but a higher tank-to-wheel compared to gasoline IC's. Some research has been
done into using special crystalline materials to store hydrogen at greater
densities and at lower pressures. A recent study by Dutch researcher Robin Gremaud
has shown that metal hydride hydrogen tanks are actually 40 to 60-percent
lighter than an equivalent energy battery pack on an electric vehicle
permitting greater range for hydrogen cars.
DISADVANTAGES
• Fuelling
fuel cells is still a problem since the production, transportation,
distribution and storage of hydrogen is difficult.
•
Reforming hydrocarbons via reformer to produce hydrogen is technically
challenging and not clearly environmentally friendly.
• The
refueling and the starting time of fuel cell vehicles are longer and the
driving range is shorter than in a “normal” car.
• Fuel
cells are in general slightly bigger than comparable batteries or engines.
• Fuel
cells are currently expensive to produce, since most units are hand-made.
• Some
fuel cells use expensive materials.
• The
technology is not yet fully developed and few products are available.
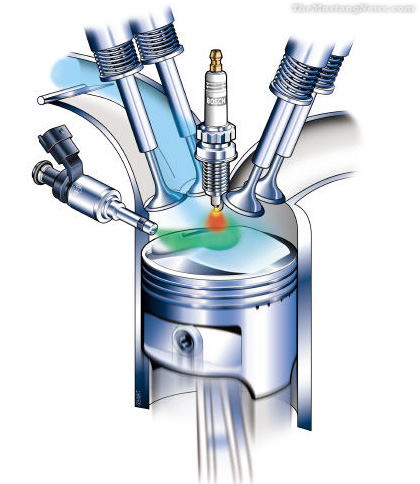
CONSTRUCTION OF HYDROGEN INJECTOR PLUG
The
hydrogen gas injector plug is used for generating hydrogen gas from ambient air
drawn into the piston chamber of an engine. The plug comprises an array of
nozzles fabricated from one or a mixture of metals. When heated to a
pre-determined temperature, the metallic nozzles become “white-hot” and
generate
photons
that react with atmospheric water to disassociate hydrogen gas there from. The
nozzle is designed so that no reaction will occur if the temperature is below
350° F. The hydrogen gas is injected from the nozzles into the piston chamber
and mixed with air. An ignition device ignites the mixture of hydrogen and air
so that it burns to power the piston in a conventional manner. The metallic
nozzle is heated via a copper conductor connected to a source of electric
current. The electrical conductors are disposed at the first end of the heat
insulating member. There are totally 9 nozzles in the array. The nozzle is
fabricated of iron alloys and tungsten. The electrical conductors are
manufactured from nickel-copper alloy. The hydrogen gas injector plug is fixed
in an internal combustion engine and a spark plug is mounted on the piston
chamber. Accordingly, it presents a hydrogen gas generator capable of
generating small amounts of hydrogen gas from water available in the atmosphere
BRIEF DESCRIPTION OF THE DRAWINGS
A
piston having rings is housed within chamber. The head and the cylinder wall
are surrounded by coolant chamber. Conventional intake and exhaust valves (not
shown) are also disposed on head. The structure and arrangement of the
aforementioned items are conventional and are not part of this new concept. A
hydrogen gas generator injector plug and a conventional spark plug are mounted
on head. Injector plug and conventional spark plug each have proximate ends
disposed within cylinder chamber.
20- Hydrogen injector plug
22- Circular chamber
22a-
Proximate end of chamber
22b-
Distal end of chamber
24-
Insulation chamber
26- Passageway
28- Cavity
30- Nozzle chamber
34- Electric current conductor
34a- Lower
end of conductor
34b- Upper
end of conductor
36- Four electric conductors
FIG.
2 is a side, sectional view of a hydrogen gas generator injector plug according
to the present invention.
ASSEMBLY OF HYDROGEN INJECTOR PLUG USING
PRO-E
As
in diagram the hydrogen gas generator injection plug comprises a circular
member fabricated from steel and having a threaded proximate end and a distal
end. A passageway extends through member from the distal end to proximate end.
An insulation member is disposed in the passageway. Insulation member is
fabricated from a material having high temperature insulation characteristics.
Member has a first end that is disposed adjacent to, but spaced slightly
inwardly of proximate end. The second end of insulation member extends above
the distal end of member. A passageway extends through insulation member from
second end and terminates at a cavity, which cavity is formed in the first end
of insulation member. A nozzle member is positioned in cavity. Nozzle member is
fabricated from a metal or a metal mixture that has the ability to react with
water at relatively high temperatures to oxidize and generate hydrogen gas.
Nozzle member must also have the ability to become “white hot” very quickly
when an adequate electrical current is applied thereto. As currently
contemplated, iron, certain alloys of iron and tungsten exhibit the requisite
abilities needed to function as the nozzle member. It should be noted however,
that any metal or alloy could be utilized if suitable. An array of hydrogen gas
injector passages is formed in nozzle member. It has been determined that nine
injector passages will provide an adequate amount of hydrogen gas to power
piston. An electric current conductor, preferably fabricated from copper,
extends through passageway. The lower end of conductor abuts nozzle member. The
upper end defines a terminal for an electrical connection. Four electrical
conductors abut the second end of member and are evenly spaced there around.
Conductors are fabricated from a nickel-copper alloy.
WORKING
In
use, ambient air containing water vapor is drawn into the cylinder chamber via
conventional intake valves (not shown) on the intake stroke. A portion of the
air water vapor mixture is forced into injector passages on the compression
stroke. An electric current is applied to nozzle member via conductor during
the latter part of the compression stroke, heating the nozzle member to a
“white-hot” temperature. The water vapor reacts with the metal in nozzle member
to oxidize the metal and produce hydrogen gas. The hydrogen gas expands through
the passages back into the cylinder chamber where it mixes with the oxygen in
the air. Spark plug is fired to
ignite the mixture, which mixture burns thereby creating a high gas pressure to
drive the piston.
Occasionally
(and in some climates), the humidity may be very low and the ambient air will
not contain enough water vapor to produce an adequate amount of hydrogen gas to
properly drive the piston. In such instances additional water vapor is injected
into the intake valve.
ADVANTAGES OF HYDROGEN GAS
Wide Range of
Flammability
Hydrogen has a wide flammability range in
comparison with all other fuels. As a result, hydrogen can be combusted in an
internal combustion engine over a wide range of fuel-air mixtures. A
significant advantage of this is that hydrogen can run on a lean mixture. A
lean mixture is one in which the amount of fuel is less than the theoretical,
stoichiometric or chemically ideal amount needed for combustion with a given
amount of air. This is why it is fairly easy to get an engine to start on
hydrogen.
Generally, fuel economy is greater and the
combustion reaction is more complete when a vehicle is run on a lean mixture.
Additionally, the final combustion temperature is generally lower, reducing the
amount of pollutants, such as nitrogen oxides, emitted in the exhaust. There is
a limit to how lean the engine can be run, as lean operation can significantly
reduce the power output due to a reduction in the volumetric heating value of
the air/fuel mixture.
Low Ignition
Energy
Hydrogen has very low ignition energy. The
amount of energy needed to ignite hydrogen is about one order of magnitude less
than that required for gasoline. This enables hydrogen engines to ignite lean
mixtures and ensures prompt ignition.
Unfortunately, the low ignition energy means that hot gases and
hot spots on the cylinder can serve as sources of ignition, creating problems
of premature ignition and flashback. Preventing this is one of the challenges
associated with running an engine on hydrogen. The wide flammability range of
hydrogen means that almost any mixture can be ignited by a hotspot.
High
Auto-ignition Temperature
Hydrogen has a relatively high autoignition
temperature. This has important implications when a hydrogen-air mixture is
compressed. In fact, the autoignition temperature is an
important factor in determining what compression ratio an engine can use, since
the temperature rise during com\pression is related to the compression ratio.
The temperature rise is shown by the
equation:
Where:
V1/V2 = the compression
ratio
T1 = absolute initial temperature
T2 = absolute final temperature
γ =
ratio of specific heats
The temperature may not exceed hydrogen’s
auto-ignition temperature without causing premature ignition. Thus, the
absolute final temperature limits the compression ratio. The high auto-ignition
temperature of hydrogen allows larger compression ratios to be used in a
hydrogen engine than in a hydrocarbon engine.
This higher compression ratio is important
because it is related to the thermal efficiency of the system .On the other
hand, hydrogen is difficult to ignite in a compression ignition or diesel
configuration, because the temperatures needed for those types of ignition are
relatively high.
High Flame Speed
Hydrogen has high flame speed at
stoichiometric ratios. Under these conditions, the hydrogen flame order of
magnitude higher (faster) than that of gasoline. This means that hydrogen
engines can more closely approach the thermodynamically ideal engine cycle. At
leaner mixtures, however, the flame velocity decreases significantly.
Low Density
Hydrogen has very low density. This results
in two problems when used in an internal combustion engine. Firstly, a very
large volume is necessary to store enough hydrogen to give a vehicle an
adequate driving range. Secondly, the energy density of a hydrogen-air mixture,
and hence the power output, is reduced. speed is nearly an order of magnitude
higher (faster) than that of gasoline. This means that hydrogen engines can
more closely approach the thermodynamically ideal engine cycle. At leaner
mixtures, however, the flame velocity decreases significantly.
AIR/FUEL
RATIO
The theoretical or stoichiometric combustion
of hydrogen and oxygen is given as:
2H2 + O2 =
2H2O
Moles of H2 for complete combustion = 2 moles
Moles of O2 for complete combustion = 1 mole
Because air is used as the oxidizer instead oxygen, the
nitrogen in the air needs to be included in the calculation:
Moles of N2 in air =
Moles of O2 (79%N2in air / 21% O2 in air)
=
1 mole of O2 x (79% N2 in air / 21% O2 in air)
= 3.762 moles N2
Number of moles of air =
Moles of O2 + moles of N2
= 1 + 3.762
= 4.762 moles
of air
Weight of O2 =
1 mole of O2 x 32 g/mole
= 32 g
Weight of N2 =
3.762 moles of N2 x 28 g/mole
=105.33 g
Weight of air =
weight of O2 + weight of N (1)
= 32g + 105.33 g
= 137.33 g
Weight of H2 =
2 moles of H2 x 2 g/mole
= 4 g
Stoichiometric air/fuel (A/F) ratio for hydrogen and air
is:
A/F based on mass =mass
of air/mass of fuel
=
137.33 g / 4 g
= 34.33:1
A/F based on volume =
volume (moles) of air/volume (moles) of fuel
= 4.762 / 2
= 2.4:1
The percent of the combustion chamber occupied by
hydrogen for a stoichiometric mixture:
% H2 = volume
(moles) of H2/total volume (2)
= volume H2/(volume air +
volume of H2)
= 29.6%
These calculations show the stoichiometric or
chemically correct A/F ratio for the complete combustion of hydrogen in air is
about 34:1 by mass. This means that for complete combustion, 34 pounds of air
are required for every pound of hydrogen. This is much higher than the 14.7:1
A/F ratio required for gasoline.
Since hydrogen is a gaseous fuel at ambient conditions it
displaces more of the combustion chamber than a liquid fuel. Consequently less
of the combustion chamber can be occupied by air. At stoichiometric conditions,
hydrogen dis-places about 30% of the combustion chamber, compared to about 1 to
2% for gasoline.
For lean A/F ratios, phi will be a value less than one. For
example, a phi of 0.5 means that there is only enough fuel available in the
mixture to oxidize with half of the air available. Another way of saying this
is that there is twice as much air available for combustion than is
theoretically required.
THERMAL EFFICIENCY
The theoretical thermodynamic efficiency of
an Otto cycle engine is based on the compression ratio of the engine and the
specific-heat ratio of the fuel as shown in the equation:
Where:
V1/V2 = the compression
ratio
γ = ratio of specific heats
ηth = theoretical thermodynamic
efficiency
If the compression ratio and the specific
heat ratio are higher, then the indicated thermodynamic efficiency too would be
higher. The compression ratio limit of an engine is based on the fuel’s
resistance to knock. A lean hydrogen mixture is less susceptible to knock than
conventional gasoline and therefore can tolerate higher compression ratios.
The specific-heat ratio is related to the fuel’s molecular structure.
The less complex the molecular structure, the higher the specific-heat ratio.
Hydrogen (γ = 1.4) has a much simpler molecular structure than gasoline and
therefore its specific-heat ratio is higher than that of conventional gasoline
(γ = 1.1).
EMISSIONS
The combustion of hydrogen with oxygen
produces water as its only product:
2H2 + O2 = 2H2O
The combustion of hydrogen with air however
can also pro-duce oxides of nitrogen (NOx):
H2 + O2 + N2 = H2O + N2 + NOx
The oxides of nitrogen are created due to the
high temperatures generated within the combustion chamber during combustion.
This high temperature causes some of the nitrogen in the air to combine with
the oxygen in the air.
The amount of NOx formed depends on:
•
The air/fuel ratio
•
The engine compression ratio
•
The engine speed
•
The ignition timing
•
Whether thermal dilution is utilized
In addition to oxides of nitrogen, traces of
carbon monoxide and carbon dioxide can be present in the exhaust gas, due to
seeped oil burning in the combustion chamber.
Depending on the condition of the engine
(burning of oil) and the operating strategy used (a rich versus lean air/fuel
ratio), a hydrogen engine can produce from almost zero emissions (as low as a
few ppm) to high NOx and significant carbon monoxide emissions.
POWER OUTPUT
The theoretical maximum power output from a
hydrogen en-gine depends on the air/fuel ratio and fuel injection method used.
As mentioned in Section 3.3, the
stoichiometric air/fuel ratio for hydrogen is 34:1. At this air/fuel ratio,
hydrogen will displace 29% of the combustion chamber leaving only 71% for the
air. As a result, the energy content of this mixture will be less than it would
be if the fuel were gasoline (since gasoline is a liquid, it only occupies a
very small volume of the combustion chamber, and thus allows more air to
enter).
Since both the carbureted and port injection
methods mix the fuel and air prior to it entering the combustion chamber, these
systems limit the maximum theoretical power obtain-able to approximately 85% of
that of gasoline engines. For direct injection systems, which mix the fuel with
the air after the intake valve has closed (and thus the combustion chamber has
100% air), the maximum output of the engine can be approximately 15% higher
than that for gasoline engines.
Therefore, depending on how the fuel is
metered, the maxi-mum output for a hydrogen engine can be either 15% higher or
15% less than that of gasoline if a stoichiometric air/fuel ratio is used.
However, at a stoichiometric air/fuel ratio, the combustion temperature is very
high and as a result it will form a large amount of nitrogen oxides (NOx),
which is a criteria pollutant. Since one of the reasons for using hydrogen is
low exhaust emissions, hydrogen engines are not normally designed to run at a
stoichiometric air/fuel ratio.
Typically hydrogen engines are designed to use about twice as much
air as theoretically required for complete combustion. At this air/fuel ratio,
the formation of NOx is reduced to near zero. Unfortunately, this also reduces
the power out-put to about half that of a similarly sized gasoline engine. To
make up for the power loss, hydrogen engines are usually larger than gasoline
engines, and are equipped with turbochargers or supercharger.
CONCLUSION
Hydrogen
being the lightest element can replace the fossil fuel and acts as a renewable energy
resources .Although the method was developed earlier hydrogen storage was
difficult and dangerous this method injecting hydrogen using the hydrogen gas
injector plug is fruitful overcoming all
the difficulties in storing hydrogen in liquid form and lead to a pollution
free atmosphere . Further the power and fuel consumption is much more better
than the conventional internal combustion engine
REFERENCES
1.Linde Division of Union Carbide
Corporation, "Survey Study of the Efficiency and Economics of Hydrogen
Liquefaction," Contract NAS 1-13395, NASA, Hampton, Virginia, April 8,
1975.
|
Figure 1
|
3.Chao, R.E., and Cox, K.E., "An Analysis of Hydrogen
Production Via Closed-Cycle Schemes," THEME Conference, Miami, Florida,
March 18-20, 1974.
4.www.geocities.com/flynn/hydrofuel/
- A Comprehensive study of hydrogen as fuel
5.Alternate Fuels – Boris
and Davis