Enthalpy is the measure of heat energy in the air due to sensible heat or latent heat. Sensible heat is the heat (energy) in the air due to the temperature of the air and the latent heat is the heat (energy) in the air due to the moisture of the air. The sum of the latent energy and the sensible energy is called the air enthalpy. Enthalpy is expressed in Btu per pound of dry air (Btu/lb of dry air) or kilojoules per kilogram (kJ/kg).
Enthalpy is useful in air heating and cooling applications. Air with same amount of energy may either be dry hot air (high sensible heat) or cool moist air (high latent heat)
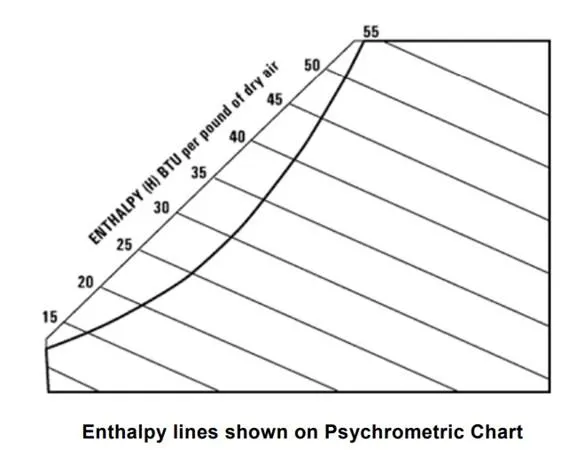
The enthalpy scale is located above the saturation, upper boundary of the chart. Lines of constant enthalpy run diagonally downward from left to right across the chart; follow almost exactly the line of constant wet bulb temperature.
When a process takes place at constant pressure, the heat absorbed or released is equal to the Enthalpy change. Enthalpy is sometimes known as “heat content”, but “enthalpy” is an interesting and unusual word, so most people like to use it. Etymologically, the word “entropy” is derived from the Greek, meaning “turning” and “enthalpy” is derived from the Greek meaning “warming”. As for pronunciation, Entropy is usually stressed on its first syllable, while enthalpy is usually stressed on the second.
Enthalpy(H) is the sum of the internal energy(U) and the product of pressure(P) and volume(V).
Enthalpy H can be written as,
H = U + pV
Where, H = Enthalpy of the system
U = Internal energy of the system
p = Pressure of the system
V = Volume of the system
Enthalpy is not measured directly, however, the change in enthalpy (ΔH) is measured, which is the heat added or lost by the system. It is entirely dependent on the state functions T, p and U.
Enthalpy can also be written as
ΔH=ΔU+ΔPV
At constant temperature, for the process heat flow(q) is equal to the change in enthalpy, this is represented as
ΔH=q
Enthalpy Units
The Enthalpy is expressed as,
H = Energy/Mass
Any physical quantity can be represented by dimensions. The arbitrary magnitudes allocated to the dimensions are called units. There are two types of dimensions namely primary or fundamental and secondary or derived dimensions.
Primary dimensions are: mass, m; length, L; time, t; temperature, T
Secondary dimensions are the ones that can be derived from primary dimensions such as velocity(m/s2 ), pressure (Pa = kg/m.s2 ).
There are two unit systems currently available SI (International System) and USCS (United States Customary System) or English system. We, however, will use SI units exclusively in this course. The SI units are based on the decimal relationship between units.







